Over the last few decades, hundreds of knee replacement devices have been recalled over serious safety risks. Three recent recalls have even inspired legal action, as injured patients and their families pursue financial compensation from manufacturers:
New knee implants have been linked to painful loosening and premature device failure. Some replacements have failed within one or two years of implantation, forcing patients to undergo risky revision procedures. Our experienced lawyers can help. Contact now for a free consultation.
Knee implants have become increasingly sophisticated in recent years, allowing surgeons to accurately mimic the feel and function of natural human joints. Today, modern replacement implants can improve mobility and cut down on recovery time, letting patients return to their normal activities quickly. It’s no wonder that over 600,000 knee replacement procedures are performed every year in the United States alone. Even more promising, knee implants have gained a striking longevity, lasting in many patients up to 20 years after implantation.
But not all knee replacement devices are created equal. In fact, a 2013 analysis from Consumer Reports found that over the previous decade, the six leading knee implant manufacturers had issued a total of 709 recalls, almost universally in response to safety risks.

Yes, there are current open recalls on the Biomet Vanguard Knee Replacement System.
Yes, our lawyers are representing individuals injured by the recalled Zimmer Biomet Vanguard Knee System. The FDA issued a recall on this product in February 2019 for a part called the Series-A Standard Patella or the Regenerex Three Peg Series-A Patella.
According to the manufacturers’ literature, a knee replacement should last 20 years. However, when recalls are issued or issues are discovered with a defective implant, we see failures regularly occurring anywhere from 2-10 years after implantation.
Some knee implant components are made with a titanium coating. This can be problematic as titanium can leach toxins into the body causing serious conditions. Others are made of cobalt-chromium which also have potential to cause metalossis and severe conditions.
Two of the largest implant manufacturers in the country, DePuy Synthes and Zimmer Biomet, account for nearly 71% of the recalls issued between 2003 and 2013, according to Consumer Reports.
Neither company offers a warranty to reimburse patients who are implanted with defective implants, even though the vast majority of knee implants reach the market with little to no FDA oversight. In large part, this lack of federal supervision explains the extraordinarily high rate of recalls in the industry. Many recalled knee implants are simply defective in design.
Even so, knee implant companies continue to introduce new “innovative” products to the healthcare market. In recent years, DePuy Synthes and Zimmer Biomet released two new replacement devices that promised to improve patient mobility and reduce complications like never before:
Both manufacturers made (and continue to make) big claims about their new devices. In speaking about the Persona implant on its website, Zimmer Biomet comes off as positively giddy, claiming to have “redefin[ed] knee arthroplasty” with an innovation that will “usher[…] in a new era of personalization.” DePuy Synthes, pushing its “innovative [and] comprehensive” Attune Knee System, sounds similar:
“the Attune Knee is one of the largest research and development projects in the history of DePuy Synthes Joint Reconstruction and combines the latest in design, kinematics, engineering and materials to deliver stability and motion.”
Surgeons, understandably, thought a revolution was coming.
For individuals over the age of 60, knee replacements are a fairly common occurrence. Over years of wear and tear and sometimes the onset of osteoarthritis or other degenerative bone conditions, these joints can begin to fail. The corrective action taken by most is to trust the medical devices manufactured by knee replacement companies with total knee replacements and this is usually done in both knees.
The manufacturer of the Vanguard knee system, Zimmer Biomet, specifically states on its website that their knee system promises to “allow surgeons to provide tailored patient care,” and to improve knee stability. They continue to state that it offers “complete component interchangeability” and contains several components to mimic the natural function of a normal healthy knee.
The FDA issued a recall on this knee replacement system in February 2019, and more specifically the “Regenerex Three Peg Series-A Patella” after several reported serious injuries where the pegs on the Patella fractured and migrated through the knee. The result is extreme pain and the need for surgery to remove the fractured component from the body.
Other concerns our firm is investigating regarding this specific Regenerex Patella knee system:
The reality was a little different. Only three years after its FDA approval, a major component of the Persona Knee was recalled. Tracking side effect reports, Biomet found that the implant was linked to an unacceptably-high rate of failure. Corporate investigations ultimately blamed the “trabecular metal tibial plate,” a component that fits on top of the shin bone and serves as a support for the implant’s remaining parts. A problem with this plate, Biomet concluded, was allowing knee implants to loosen from their proper placement, detaching from the bone entirely. The implants, due to a defect, were migrating inside the body.
Loosening is a primary contributor to total knee implant failure, according to New York’s Hospital for Special Surgery. It can also be extremely painful and often leads to a significant reduction in mobility, preventing patients from engaging in their daily activities.
The most serious consequence of an implant failure is revision surgery, in which patients are forced to undergo a second invasive procedure to have their implant replaced.
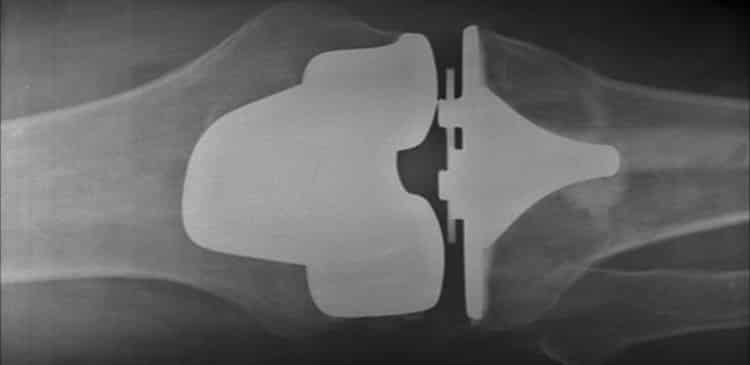
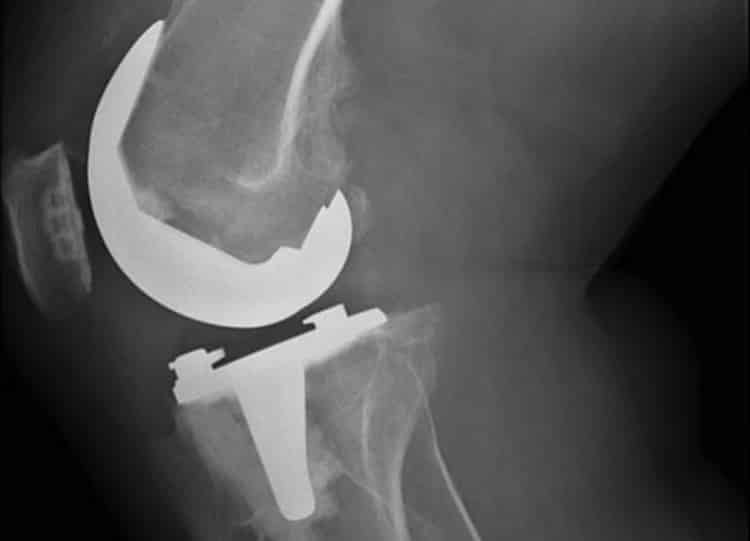
True to form, Zimmer Biomet’s Persona implant had been linked to a shocking number of revision procedures. After analyzing product complaints, the company determined that fully 38% of patients had already undergone revision procedures for device loosening or displayed symptomatic radiolucent lines, a gap between the implant and bone that appears on an X-ray. Radiolucent lines, especially when accompanied by pain, swelling or other symptoms, is usually a harbinger of device loosening and total implant failure.
In light of these risks, Zimmer Biomet redesigned the Persona Knee and quickly returned their product to the market. The implant’s problems, though, don’t seem to be over. The US Food & Drug Administration continues to receive complaints of premature loosening, at times within one or two years of implantation.
Biomet isn’t alone in courting controversy. In fact, the company’s direct competitor, DePuy Synthes, also ran into serious problems in 2015. That year, a full five years after the Attune Knee System was approved, DePuy realized that one of the implant’s components posed a significant safety risk.
Before a knee implant can be inserted, surgeons have to measure the patient’s bones and range of motion to properly calibrate the final device. To do so, doctors use a “trial” implant, testing the patient’s natural joint before drilling any holes into the bone. The trial implant that DePuy supplied along with its Attune Knee System, however, featured a small wire spring that easily became damaged and fell out during use. If that happened, the spring could fall directly into the patient’s surgical site and be forgotten by an unknowing surgeon.
Just like Zimmer Biomet, DePuy issued a recall for the Attune Knee System’s articulation surface and modified its design. Complaints, however, haven’t ended. In a growing number of side effect reports, patients and surgeons say the Attune replacement knee can loosen prematurely, causing severe pain, decreased mobility and, in some cases, device failure.
Undergoing a knee revision procedure is, for many patients, only the beginning of a long and traumatic process of recovery. As the American Academy of Orthopaedic Surgeons notes, revision procedures are “longer” and “more complex” than initial knee replacements, “requir[ing] extensive planning, and specialized implants and tools to achieve a good result.” Due to its length and complexity, knee revision surgery also carries a higher risk of complication, including:
We don’t think victims should be forced to pay for the complications of a defective medical device. Families shouldn’t be asked, during their worst moments of grief, to cover the costs of medical care and funeral services when their loved one’s death was caused by a corporation’s total disregard for patient safety. Thankfully, America’s strong tradition of product liability law allows injured patients and their families to pursue financial compensation.
Knee implant patients who received eligible replacement devices and suffered serious personal injuries may be eligible to file a lawsuit, demanding compensation and accountability from medical device manufacturers.
Contrary to popular opinion, medical device lawsuits are not an example of medical malpractice litigation. Instead, claims filed against companies who make or sell defective implants are almost always governed by the legal doctrine of strict liability. In a malpractice lawsuit, patients must prove that their doctor or surgeon was negligent in some way. Most product liability lawsuits, on the other hand, don’t work like that.
It’s not necessary to prove that the manufacturer was negligent in designing or manufacturing the medical device. Injury victims will only need to demonstrate that their implant was defective in some way, that it left the manufacturer in a condition that posed unreasonable danger to patients. As we’ve seen, many of the nation’s most prominent knee implants already seem to fit the bill. While a manufacturer’s recall is not automatic proof that a product is defective, it certainly makes the existence of a defect more likely.
There is, however, a wrinkle to medical device cases that should be taken into account. The defendant, in this case the medical device manufacturer, usually has the legal right to inspect the medical device, looking for ways in which the product was modified after sale or evidence that the implant’s failure was due to natural causes. It’s a key point for patients who have yet to undergo revision procedures. Think your medical device is failing? Tell your doctors and surgeon to keep the implant intact and unchanged. Ask well before the date of your surgery; the medical team will need to make arrangements for storing and preserving the device.
This can present a problem for some medical device patients, especially knee implant patients who have already undergone a revision procedure. Most surgeons don’t return devices to patients after removing them. In many cases, the implant is simply disposed of as waste. Cases have been dismissed without access to the medical device at issue.
Despite this hurdle, some patients will still be able to file suit, even though their implants were thrown out. Doctors often take pictures of medical devices after removal, especially when they suspect that a product failure led to patient harm. Detailed records of the device’s condition after removal may also help to buttress a case.
If you’re reading this page, you probably saw a television commercial about filing a knee implant lawsuit. A number of law firms, over the last few months, have started producing these ads, which usually run on local stations. In many commercials, attorneys mention two medical device manufacturers in particular, Zimmer Biomet and DePuy Synthes, the country’s leading makers of knee replacement devices.
The TV ads usually say that knee implants made by both Biomet and DePuy have been linked to unacceptably high rates of failure; some commercials refer to a specific complication known as “loosening.” This is what happens when a knee replacement, usually bolted or cemented to a patient’s shin bone, becomes detached.
Needless to say, implant loosening can lead to severe complications.
As the American Academy of Orthopaedic Surgeons notes, loosening is a primary contributor in most device failures; patients can begin to experience extreme pain, swelling and significant decreases in mobility and function. Most people with a loose knee replacement will need to undergo a costly revision procedure, which comes with its own significant risks.
Revision surgery is more dangerous than initial knee implant placement, largely because removing a broken implant and replacing it with a new one takes much longer and requires specialized equipment. The procedure’s complexity increases complication rates.
Some patients develop blood clots, which can become life-threatening if they migrate to the heart or lungs. Others suffer nerve damage, while many will have to deal with excessive bleeding. Surgical infections are also possible, as is the case for all invasive operations. It should also be noted that people who get knee replacements are often older and already dealing with complex medical problems, a fact that may increase their risk for surgical complications even further.
In reality, many knee implant patients should never have to undergo a revision procedure. Today’s most advanced devices are expected to last at least 20 years without much wear or damage.
In 2014, 4.7 million Americans were walking around with artificial knees, allowing patients to remain mobile despite severe arthritis and other medical conditions. Most of these patients are over the age of 65. Knee replacement is one of the most common surgical procedures performed in the US and represents a huge patient population for medical device manufacturers to capitalize on. The knee implant industry, however, is not known for putting patient safety first.
Over the last 15 years, device manufacturers have issued over 700 recalls for knee replacement products. More than 7 out of every 10 of these recalls were announced by DePuy Synthes and Zimmer Biomet. In a wave of recent product liability lawsuits, patients say two knee replacement devices are particularly dangerous.
The Persona Knee, approved in 2012, was hailed by its manufacturer Zimmer Biomet as a literal “redefinition” of knee replacement surgery that would “usher […] in a new era of personalization.” DePuy Synthes had made similar claims about its “innovative” Attune Knee System, released in 2010. Both companies said that their revolutionary new products would allow surgeons to mimic the form and function of natural joints more accurately, in part due to precision sizing. Then, both implants were recalled.
Technically, Biomet recalled only one component of the Persona Knee, the device’s “trabecular metal tibial plate,” which sits directly on top of the shin bone. Biomet had learned, after tracking patient side effect reports, that the plate’s design increased the risk of implant loosening and failure. DePuy took a similar line, recalling the Attune Knee System’s “articulation surface instruments” in 2015. This component, featured in the implant’s accompanying “trial” device, had been outfitted with a small wire spring that could fall off during sizing tests, drop into the surgical site and cause severe complications if left inside the patient.
In both cases, the designs were modified, but many patients believe that these knee implants are still defective. Reports continue to surface, linking the Persona knee to premature failures, some occurring only one year after placement. Similar complaints about the Attune Knee System are being leveled against DePuy.
Did you or a loved one undergo a knee revision surgery after an implant loosened, disconnected from the bone or failed completely? Contact our experienced medical device attorneys today to receive a free consultation. You can learn more about your legal options at no cost and no obligation. Please note that time may be limited. Every state has a law, known as the statute of limitations, that restricts the amount of time injured patients and families have to file suit. Act now; before your rights are lost.
 info@legalherald.com
info@legalherald.com